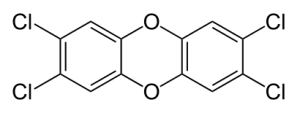
2,3,7,8-Tetrachlorodibenzo-p-dioxin, referred to as dioxin, is a contaminant in Agent Orange. It is toxic and known to cause developmental defects and cancer.
From Science Daily:
Since the 1960s, when the defoliant Agent Orange was widely used in Vietnam, military, industry and environmental groups have debated the toxicity of one of its ingredients, the chemical dioxin, and how it should be regulated.
But even if all the dioxin were eliminated from the planet, Washington State University researchers say its legacy would live on in the way it turns genes on and off in the descendants of people exposed over the past half century.
Writing in the journal PLoS ONE, biologist Michael Skinner and members of his lab say dioxin administered to pregnant rats resulted in a variety of reproductive problems and disease in subsequent generations. The first generation of rats had prostate disease, polycystic ovarian disease and fewer ovarian follicles, the structures that contain eggs. To the surprise of Skinner and his colleagues, the third generation had even more dramatic incidences of ovarian disease and, in males, kidney disease.
“Therefore, it is not just the individuals exposed, but potentially the great-grandchildren that may experience increased adult-onset disease susceptibility,” says Skinner.