Today’s science or bullshit is brought to you by food republic in a post titled “6 Scary Things Hiding in Your Food”. Excerpt:
These days it’s hard for even die-hard foodies to know what they’re eating or drinking. That’s because food has changed from something that didn’t need a modifier – if it walked, swam, flew or grew out of the ground, it was food – to something that stopped off at Mr. Burns’ nuclear plant on the way to your plate.
Let’s call it “foodiness.” Like Stephen Colbert’s truthiness, which wasn’t about truth, we’re not consuming food as much as we’re consuming an edible manufactured doppelganger designed to look and taste like food, but isn’t actually food: like veggie puffs with no vegetables; fruit bars with no fruit; like gold fish crackers with no goldfish.
First up on the list of six is TBHQ.
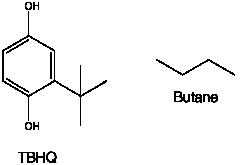
1. TBHQ, a.k.a Butane
Turns out Butane isn’t just for lighters anymore – it’s also an artificial anti-oxidant that they put it in chicken nuggets to keep them “fresh” tasting.
TBHQ is NOT butane. TBHQ has the chemical formula C10H14O2 & is a tan or brown powder at room temperature. Butane has the formula C4H10 and is a colorless odorless gas. Take a look at the structures of the molecules and see that there is absolutely no similarity.
And for the record TBHQ is safe and approved by the FDA as a preservative and food additive when its less than 0.02% of the oil or fat content in foods. Next up on the list is estrogen.
2. Estrogen:
Regular milk is full of hormones used by the milk industry to keep the cows knocked up and lactating all year round. Sound gross? It is. So when you drink regular milk you take a shot of hormones with it. And all you wanted was a bowl of cereal.
Found in: All non-organic dairy.
Fair enough. Estrogen is a hormone and cows are pumped full of hormones so they keep lactating year round. But there isn’t really much difference in the amount of estrogen in regular milk when compared to the estrogen levels in organic dairy. And in milk from any animal there are going to multiple hormones, its unfair to single out estrogen, when their are plenty others, even though none have been scientifically linked to poor health outcomes. Hormone free milk doesn’t exist.
And theres more:
4. Propylene glycol, a.k.a antifreeze:
Uhmm, she has the wrong glycol here. Propylene glycol is in fact quite harmless. It is sometimes used as a nontoxic antifreeze. It is metabolized in humans into compounds that are normally found in digestive pathways. Serious toxicity isn’t observed in humans unless you ingest a whole hell of a lot in a very short time. Long term exposure to the amounts usually found in food is also not thought you cause any deleterious health effects. She is thinking of ethylene glycol which is much more toxic and used as antifreeze. When ethylene glycol is metabolized the end result is oxalic acid, which is toxic and effects the central nervous system, the heart and the kidneys. Needless to say you should never ingest ethylene glycol. If you see it listed in the ingredients of anything you eat, put it back on the shelf or throw it away.
So you heard it here folks. This is definitely bullshit and not science. Here’s some links if you want to read more.
References:
TBHQ is safe for food use. [US Law]
Hormones in Milk [PubMed]
Propylene glycol toxicity [US EPA, PDF]